Targeting solid cancer with next-generation TCR therapies directed against mutations that are only found in the patient’s tumor
Our Science
Targeting solid cancer with a novel T cell receptor therapy (TCR-T) approach.
Engineered T cell therapies have demonstrated great benefit to patients with some difficult-to-treat cancers. These therapies have transformed the treatment paradigm for some patients with blood cancers, however, a large unmet need remains for patients with advanced solid tumors – driven in part by the availability of only a small number of safe and broadly expressed drug targets. Neogene leverages its portfolio of tumor-specific TCRs targeting hotspot mutations in KRAS and TP53, its proprietary TCR identification and selection platform and T cell engineering technologies to overcome these limitations.
Neoantigens represent attractive targets for T cell therapies.
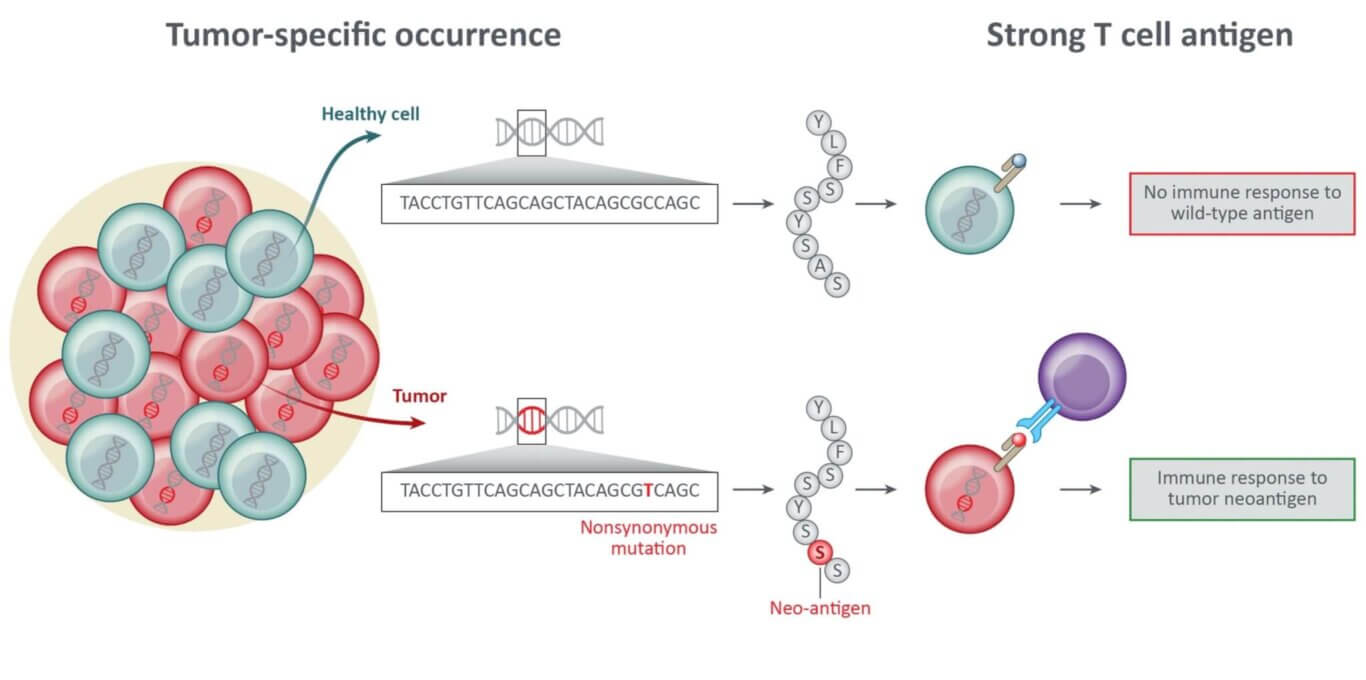
The development and evolution of cancer is driven by the accumulation of DNA mutations in cancerous cells. Some of the DNA mutations that arise in cancer cells can generate antigens with an altered amino acid sequence, which are known as neoantigens. Given their exclusive occurrence in the tumor, neoantigens represent safe targets for cancer therapy. Importantly, neoantigens can render the tumor “visible” to T cells of the immune system and T cells that target neoantigens have been identified in patients experiencing regression of advanced metastatic tumors.
The spectrum of neoantigens expressed by tumors differs between patients, even for those suffering from tumors of the same histological origin. Furthermore, the majority of neoantigens found in a given patient’s tumor are unique to that patient and are referred to as patient-specific neoantigens. However, a minority of neoantigens, such as those arising from driver mutations in oncogenes such TP53 and KRAS, are shared between some patients in a wider variety of cancers, and these are referred to as shared neoantigens.
Our APPROACH
Neogene’s portfolio of TCRs targeting shared neoantigens in combination with its proprietary neoantigen TCR identification and selection platform and T cell engineering technologies deliver an industry-leading approach to develop two classes of neoantigen-targeted TCR-Ts for patients with solid tumors:
- TCR-Ts consisting of T cells engineered to express a shared neoantigen-specific TCR
- TCR-Ts consisting of T cells engineered to express TCRs recognizing patient-specific tumor mutations (fully individualized engineered T cell therapies)
T cell therapies targeting shared neoantigens expressed on a variety of solid tumors
Through our exclusive license with the National Cancer Institute (NCI), Neogene has a portfolio of shared neoantigen-specific TCR genes targeting hotspot mutations in KRAS and TP53 driver oncogenes. Two of these TCRs combined with Neogene’s T cell engineering technologies form the basis of Neogene’s first shared neoantigen-specific TCR-Ts, NT-175 and NT-112. Alongside this, Neogene is using its proprietary neoantigen TCR identification and selection platform for the discovery of novel shared neoantigen-specific TCRs for potential future clinical development.
Fully individualized T cell therapies targeting patient-specific mutations
Our fully individualized T cell therapies are based on our proprietary TCR identification and selection platform as well as our T cell engineering technologies that enable the generation of a multi-specific T cell therapy containing more than one TCR to simultaneously targeted different neoantigens within a given tumor. The multi-specificity of our fully individualized TCR therapies aims to reduce the possibility for therapy-induced resistance mechanisms and increase the strength and duration of the therapeutic response.
Our Pipeline
We are developing a unique and growing pipeline of fully-individualized and shared neoantigen-targeting TCR therapies with the goal of changing the paradigm of solid cancer treatment.
Program
Type
Pre-clinical
Phase 1
Phase 2
Phase 3
NT-125
An autologous, fully-individualized TCR therapy that derives neoantigen-specific TCRs from a patient’s tumor infiltrating T cells, identified using Neogene’s proprietary neoantigen-specific TCR identification and selection platform.
NT-175
An autologous, TP53 R175H-targeting, HLA-A*02:01 specific TCR therapy with T cells that are armored through the genetic knock-out of a critical regulator of T cell activity
This product is currently being tested in a Phase I trial in the US: NCT05877599
NT-112
An autologous, KRAS G12D-targeting, HLA-C*08:02 specific TCR therapy with T cells that are armored through the genetic knock-out of a critical regulator of T cell activity
This product is currently being tested in a Phase I trial in the US: NCT06218914
OUR EXPANDNED ACCESS POLICY
Neogene Therapeutics, Inc., a member of the AstraZeneca group, is focused on the development of novel therapies to treat cancer.
At this time, Neogene Therapeutics does not provide access to investigational products outside of clinical trials.
We encourage patients to participate in clinical trials of our investigational therapies. Clinical trials are designed, conducted, and monitored to evaluate the safety and efficacy of investigational therapies before they are submitted to regulatory agencies for review and with the intent to make them more broadly available to patients.
You and your health care provider may learn more about our clinical trials by going to the pipeline section of our website or by visiting www.clinicaltrials.gov and searching for Neogene Therapeutics.
If you are a health care provider who is interested in learning more about one of our investigational therapies, or a physician with questions about participation in one of our clinical trials, please submit a request to medicalaffairs@neogene.com.
Neogene Therapeutics reserves the right to revise this expanded access policy at any time.